Uh-oh, your gonna get Professor PC started again

too late.....he did.....headlights and taillights.....thats a good one....only a good excuse for 90 years or so though.....red and white lights were seen long before lights were invented i'm afraid....and what about the other 6 colors that are seen regularly.....one weekends observations does not make a solid conclussion...especially one as far fetched as car lights.....now thats funny....the red is because of altitude and oxygen.....not a chevy....sorry....just like a huge tsunami wave is not made from wind and storms but rather an event underground not even related to the sea fer cry eye out loud....here read this
2) What makes the color of the aurora?
The composition and density of the atmosphere and the altitude of the aurora determine the possible light emissions.
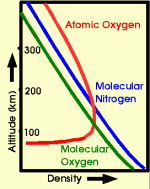
When an excited atom or molecule returns to the ground state, it sends out a photon with a specific energy. This energy depends on the type of atom and on the level of excitement, and we perceive the energy of a photon as color. The upper atmosphere consists of air just like the air we breathe. At very high altitudes there is atomic oxygen in addition to normal air, which is made up of molecular nitrogen and molecular oxygen. The energetic electrons in aurora are strong enough to occasionally split the molecules of the air into nitrogen and oxygen atoms. The photons that come out of aurora have therefore the signature colors of nitrogen and oxygen molecules and atoms. Oxygen atoms, for example, strongly emit photons in two typical colors: green and red. The red is a brownish red that is at the limit of what the human eye can see, and although the red auroral emission is often very bright, we can barely see it.
Photo by Jan Curtis
Photographic film has a different sensitivity to colors than the eye, therefore you often see more red aurora on photos than with the unaided eye. Since there is more atomic oxygen at high altitudes, the red aurora tends to be on top of the regular green aurora. The colors that we see are a mixture of all the auroral emissions. Just like the white sunlight is a mixture of the colors of the rainbow, the aurora is a mixture of colors. The overall impression is a greenish-whitish glow. Very intense aurora gets a purple edge at the bottom. The purple is a mixture of blue and red emissions from nitrogen molecules.
The green emission from oxygen atoms has a peculiar thing about it: usually an excited atom or molecule returns to the ground state right away, and the emission of a photon is a matter of microseconds or less. The oxygen atom, however, takes its time. Only after about a 3/4 second does the excited atom return to the ground state to emit the green photon. For the red photon it takes almost 2 minutes! If the atom happens to collide with another air particle during this time, it might just turn its excitation energy over to the collision partner, and thus never radiate the photon. Collisions are more likely when the atmospheric gas is dense, so they happen more often the lower down we go. This is why the red color of oxygen only appears at the very top of an aurora, where collisions between air molecules and atoms are rare. Below about 100 km (60 miles) altitude even the green color doesn't get a chance. This happens when we see a purple lower border: the green emission gets quenched by collisions, and all that is left is the blue/red mixture of the molecular nitrogen emission.
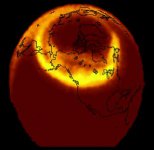
now look at how PAULDING IS AT THE VERY BASE OF AN AURORA so that you would be viewing it from the bottom of the light skirt which would produce red in abundance and white as well....more so than other colors with a ratio of 100 to 1
click on this picture and find the great lakes and see you are at the base of an aurora all the time....the brightest yellow leaning towards white hot is THE VERY BASE OF THE SKIRT
 for further investigating. We're always up for a viewing! Look forward to it!
for further investigating. We're always up for a viewing! Look forward to it!